Dr Thomas Daussmann, Jan-Dirk Spoering, Dr Marc R. Hayes, Dr Christoph Strunk,
Enzymaster Deutschland
In today’s market, chemical processing approaches must achieve product quality specifications while also achieving higher sustainability levels. Chemical manufacturers increasingly value ‘greener’ strategies. Fortunately, economy and ecology often go hand in hand. A process that can run under ambient temperature, use fewer solvents and/or generate less wastewater significantly reduces the environmental impact and costs.
The Enzymaster team has developed methods to produce the two chiral amines (R)-α-phenylethylamine (PEA) from acetophenone and (R)-(+)-1-(1-naphthyl)ethylamine (NEA) from 1-acetylnaphthalene. The processes were optimised toward high atom efficiency and overall low waste production.
Chiral amines are an essential class of molecules in the chemical and pharmaceutical industries because many drugs and drug candidates contain an amine group or functional groups derived from an amine. For example, NEA is an intermediate for synthesising the calcimimetic Cinacalcet API and PEA is used as a chiral auxiliary and chiral building block.
Recent patents describing the chemical synthesis of PEA from the ketone acetophenone use expensive transition metal catalysts, such as palladium or ruthenium. These catalysts need to be recycled efficiently to reduce losses and avoid product contamination.
Additionally, chemical catalysts often lack sufficient stereoselectivity to produce one enantiomer of the product exclusively. Therefore, to purify the desired product stereoisomer, the synthesis is followed by resolution using chiral substances such as D-lactic acid or (+)-phencyphos in the case of NEA to precipitate only the required enantiomer. This step is followed by dissolving the salt and purifying it again to yield the final product.
These strategies suffer from low E-factors because of the usage of multiple auxiliary chemicals needed for the resolution and the downstream purification, increasing the processes’ environmental impact. However, strategies dedicated to greener chemistry prioritise improving the product: waste ratio and reducing the environmental burden of the catalyst and the production process as a whole.
Especially for the synthesis of chiral substances, using enzymes as natural, bio-based catalysts is an attractive alternative. Such biocatalysts eliminate the need for transition metal catalysts and, because of their inherent selectivity, no protection, deprotection, or resolution steps are needed—hence, suitable enzymes produce large amounts of the desired stereoisomer.
Yet, nature often does not provide us with enzymes ready to withstand the rigours of the commercial production environment, especially harsh process conditions or utilising challenging non-natural substrates and/or using high substrate concentrations with low water solubility.
However, enzyme engineering advancements allow us to quickly evolve an enzyme to achieve the characteristics required for a given process. To evolve enzymes that stand up to the task, we use our proprietary directed evolution technology platform, BioEngine®, to engineer improved enzyme variants.
Specifically, for the synthesis of NEA and PEA, we used a transaminase for the asymmetric reductive amination of the keto function in the substrate molecule. However, the natural enzyme for the synthesis of PEA was inhibited at 5 g/L product concentrations and inactivated at 2 M concentrations of the necessary amine donor iso-propylamine (IPM).
These limitations are typical when using transaminases and many other enzymes in non-natural synthetic routes, as desired performance levels tend to be limited to water-based reaction conditions. For example, only low product concentrations typically result from the poorly water-soluble ketones acetophenone and 1-acetylnaphthalene when synthesising PEA and NEA in an aqueous reaction set-up, respectively. Most literature describes values of <10 g/L, resulting in a water/product content of only 1% (w/v).
Because of the low product concentrations, productivity is low and isolating the product can be challenging. Specifically, to isolate the desired amine product from the water phase, energy-intensive water distillation and/or environmentally harmful solvent extraction are required. However, possible solutions, such as substrate emulsions or high concentrations of organic co-solvents, significantly decrease enzyme stability.
To address these challenges, we increased the stability of a transaminase using our BioEngine® enzyme evolution platform. In this case, the enzyme was engineered to deliver the target performance after ten rounds of enzyme evolution.
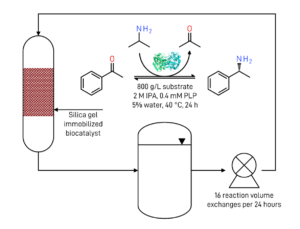 Whereas enzymes are usually applied in aqueous reaction environment, after evolution, we used the transaminase in neat substrate conditions with 800 g/L of substrate, and the enzyme was stable for more than 180 hours at 2 M IPM. No additional solvents or reagents were required for the synthesis, and very high space–time yields were obtained, with up to 160 g/L/d in the case of PEA.
Whereas enzymes are usually applied in aqueous reaction environment, after evolution, we used the transaminase in neat substrate conditions with 800 g/L of substrate, and the enzyme was stable for more than 180 hours at 2 M IPM. No additional solvents or reagents were required for the synthesis, and very high space–time yields were obtained, with up to 160 g/L/d in the case of PEA.
Overall, enzymes can eliminate costly transition metal catalysts and resource-intensive protection/ deprotection or resolution steps for the synthesis of chiral amines and numerous other fine chemicals. But wild-type enzymes are nearly always insufficient for commercial applications and must be engineered to achieve the needed performance.
Enzyme evolution is a fruitful and auspicious process to optimise enzymes to perform successfully in efficient, sustainable and more cost-effective API and fine chemical manufacturing.
Please find original article here:
https://viewer.joomag.com/speciality-chemicals-magazine-jul-aug-2022/0888142001656606623?short&